what is difference between methanol and ethanol Methanol ethanol difference
Methanol and ethanol are two types of alcohol that are commonly used as fuel, solvents, and in the production of other chemicals. While they share some similarities, there are also key differences between them that are important to understand. In this article, we will explore the differences between methanol and ethanol.
Chemical Composition
The most obvious difference between methanol and ethanol is their chemical composition. Methanol, also known as wood alcohol, has the chemical formula CH3OH, while ethanol, also known as grain alcohol, has the chemical formula C2H5OH. Methanol contains one carbon atom, one oxygen atom, and four hydrogen atoms, while ethanol contains two carbon atoms, six hydrogen atoms, and one oxygen atom.
 Despite their similar chemical structures, methanol and ethanol have different physical and chemical properties. Methanol has a boiling point of 64.7°C, while ethanol has a boiling point of 78.4°C. Methanol is also highly toxic and can cause blindness or death if ingested in large amounts, while ethanol is less toxic and is often consumed in alcoholic beverages.
Despite their similar chemical structures, methanol and ethanol have different physical and chemical properties. Methanol has a boiling point of 64.7°C, while ethanol has a boiling point of 78.4°C. Methanol is also highly toxic and can cause blindness or death if ingested in large amounts, while ethanol is less toxic and is often consumed in alcoholic beverages.
Uses
Methanol and ethanol are used for different purposes due to their different properties. Methanol is primarily used as a solvent, antifreeze, and fuel. It is also used in the production of formaldehyde, acetic acid, and other chemicals. Methanol is a popular fuel for race cars and other high-performance vehicles due to its high octane rating, which means it can generate more power than gasoline.
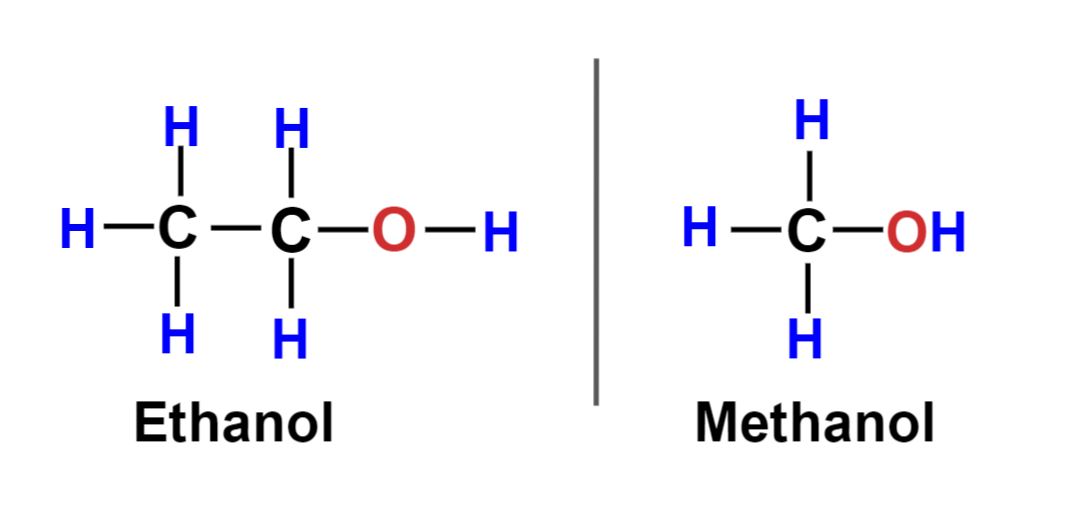 Ethanol, on the other hand, is primarily used as a fuel, solvent, and in the production of alcoholic beverages. It is also used in the production of hand sanitizers and as a renewable source of energy.
Ethanol, on the other hand, is primarily used as a fuel, solvent, and in the production of alcoholic beverages. It is also used in the production of hand sanitizers and as a renewable source of energy.
Production
Methanol and ethanol are produced by different methods. Methanol is primarily produced from natural gas, coal, and biomass, using a process called gasification. Ethanol, on the other hand, is primarily produced from corn, sugarcane, and other crops, using a process called fermentation.
Conclusion
In conclusion, while methanol and ethanol share some similarities, they are two distinct chemicals with different properties and uses. Methanol is highly toxic and primarily used as a solvent, antifreeze, and fuel, while ethanol is less toxic and primarily used as a fuel, solvent, and in the production of alcoholic beverages and hand sanitizer. Understanding the differences between these two chemicals is important for anyone who works with them or uses products that contain them.
If you are looking for Difference Between Ethanol and Methanol | Difference Between you’ve came to the right place. We have 5 Pics about Difference Between Ethanol and Methanol | Difference Between like Difference Between Ethanol and Methanol | Difference Between, 12 differences between methanol and ethanol - DewWool and also Difference Between Ethanol and Methanol | Difference Between. Here it is:
Difference Between Ethanol And Methanol | Difference Between
 www.differencebetween.netethanol methanol difference between vs
www.differencebetween.netethanol methanol difference between vs
12 Differences Between Methanol And Ethanol - DewWool
 dewwool.commethanol ethanol differences
dewwool.commethanol ethanol differences
12 Differences Between Methanol And Ethanol - DewWool
 dewwool.comethanol methanol
dewwool.comethanol methanol
Difference Between Ethanol And Methanol
 pediaa.commethanol ethanol difference
pediaa.commethanol ethanol difference
Difference Between Ethanol And Methanol
 pediaa.commethanol ethanol difference between chemical
pediaa.commethanol ethanol difference between chemical
Methanol ethanol difference. Methanol ethanol differences. Difference between ethanol and methanol